Managing Fermented Food Microbes to Control Quality
Fermented foods are produced through controlled microbial growth — but how do industry professionals manage those complex microorganisms? Three panelists, each with experience in a different field and at a different scale — restaurant chef, artisanal cheesemaker and commercial food producer — shared their insights during a TFA webinar, Managing Fermented Food Microbes to Control Quality.
“Producers of fermented foods rely on microbial communities or what we often call microbiomes, these collections of bacteria yeasts and sometimes even molds to make these delicious products that we all enjoy,” says Ben Wolfe, PhD, associate professor at Tufts University, who moderator the webinar along with Maria Marco, PhD, professor at University of California, Davis (both are TFA Advisory Board members).
Wolfe continued: “Fermenters use these microbial communities every day right, they’re working with them in crocks of kimchi and sauerkraut, they’re working with them in a vat of milk as it’s gone from milk to cheese, but yet most of these microbial communities are invisible. We’re relying on these communities that we rarely can actually see or know in great detail, and so it’s this really interesting challenge of how do you manage these invisible microbial communities to consistently make delicious fermented foods.”
Three panelists joined Wolfe and Marco: Cortney Burns (chef, author and current consultant at Blue Hill at Stone Barns in New York, a farmstead restaurant), Mateo Kehler (founder and cheesemaker at Jasper Hill in Vermont, a dairy farm and creamery) and Olivia Slaugh (quality assurance manager at wildbrine | wildcreamery in California, producers of fermented vegetables and plant-based dairy).
Fermentation mishaps are not the same for producers because “each kitchen is different, each processing facility, each packaging facility, you really have to tune in to what is happening and understand the nuance within a site,” Marco notes. “Informed trial and error” is important.
The three agreed that part of the joy of working in the culinary world is creating, and mistakes are part of that process.
“We have learned a lot over the years and never by doing anything right, we’ve learned everything we know by making mistakes,” says Kehler.
One season at Jasper Hill, aspergillus molds colonized on the rinds of hard cheeses, spoiling them. The cheesemakers discovered that there had been a problem early on as the rind developed. They corrected this issue by washing the cheese more aggressively and putting it immediately into the cellar.
“For the record, I’ve had so many things go wrong,” Burns says. A koji that failed because a heating sensor moved, ferments that turned soft because the air conditioning shut off or a water kefir that became too thick when the ferment time was off. “[Microbes are] alive, so it’s a constant conversation, it’s a relationship really that we’re having with each and every one on a different level, and some of these relationships fall to the wayside or we forget about them or they don’t get the attention they need.”
Burns continues: “All these little safeguards need to be put in place in order for us to have continual success with what we’re doing, but we always learn from it. We move the sensor, we drop the temperature, we leave things for a little bit longer. That’s how we end up manipulating them, it’s just creating an environment that we know they’re going to thrive in.”
Slaugh distinguishes between what she calls “intended microbiology” — the microbes that will benefit the food you’re creating — and “unintended microbiology” — packaging defects, spoilage organisms or a contamination event.
Slaugh says one of the benefits of working with ferments at a large scale at wildbrine is the cost of routine microbiological analysis is lower. But a mistake is stressful. She recounted a time when thousands of pounds of food needed to be thrown out because of a contaminant in packaging from an ice supplier.
“Despite the fact that the manufacturer was sending us a food-grade or in some cases a medical-grade ingredient, the container does not have the same level of sanitation, so you can’t really take these things for granted,” Slaugh says.
Her recommendations include supplier oversight, a quality assurance person that can track defects and sample the product throughout fermentation and a detailed process flow diagram. That document, Slaugh advises, should go far beyond what producers use to comply with government food regulations. It should include minutiae like what scissors are used to cut open ingredient bags and the process for employees to change their gloves.
“I think this is just an incredible time to be in fermented foods,” Kehler adds. “There’s this moment now where you have the arrival of technology. The way I described being a cheesemaker when I started making cheese almost 20 years ago was it was like being a god, except you’re blind and dumb. You’re unleashing these universes of life and then wiping them out and you couldn’t see them, you could see the impacts of your actions, but you may or may not have control. What’s happened since we started making cheese is now the technology has enabled us to actually see what’s happening. I think it’s this groundbreaking moment, we have the acceleration of knowledge. We’re living in this moment where we can start to understand the things that previously could only be intuited.”
- Published in Food & Flavor, Science
Can Microbes Eat Plastic?
Scientists found that rumen microbes, which ferment feed in a cow’s stomach and produce fatty acids, can also break down plastics, including the common polyethylene terephthalate (PET) used in food and drink packaging. Rumen microbes are found in the rumen of cows, the largest compartment of their stomach.
These researchers hope to determine the specific enzymes used by the microbes in this process, then genetically engineer the microbes to produce them in large quantities that could then be used at an industrial scale. The study, conducted by the University of Natural Resources and Life Sciences in Vienna, is published in the journal Frontiers in Bioengineering and Biotechnology.
This is not the first bacterium found to consume plastic. Ideonella sakaiensis, in enzymes secreted by some marine organisms and in certain fungi — and used in sake fermentation — also breaks down PETs.
Read more (Live Science)
- Published in Science
Fermentation for Alternative Proteins
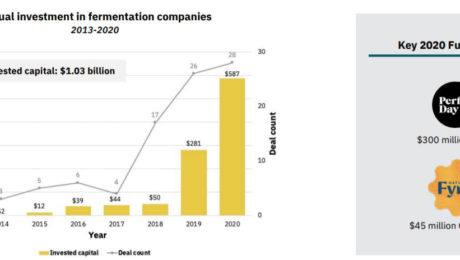
Investments in alternative protein hit their highest level in 2020: $3.1 billion, double the amount invested from 2010-2019. Over $1 billion of that was in fermentation-powered protein alternatives.
It’s a time of huge growth for the industry — the alternative protein market is projected to reach $290 billion by 2035 — but it represents only a tiny segment of the larger meat and dairy industries.
Approximately 350 million metric tons of meat are produced globally every year. For reference, that’s about 1 million Volkswagen Beetles of meat a day. Meat consumption is expected to increase to 500 million metric tons by 2050 — but alternative proteins are expected to account for just 1 million.
“The world has a very large demand for meat and that meat demand is expected to go up,” says Zak Weston, foodservice and supply chain manager for the Good Food Institute (GFI). Weston shared details on fermented alternative proteins during the GFI presentation The State of the Industry: Fermentation for Alternative Proteins. “We think the solution lies in creating alternatives that are competitive with animal-based meat and dairy.”
Why is Alternative Protein Growing?
Animal meat is environmentally inefficient. It requires significant resources, from the amount of agricultural land needed to raise animals, to the fertilizers, pesticides and hormones used for feed, to the carbon emissions from the animals.
Globally, 83% of agricultural land is used to produce animal-based meat, dairy or eggs. Two-thirds of the global supply of protein comes from traditional animal protein.
The caloric conversion ratios — the calories it takes to grow an animal versus the calories that the animal provides when consumed — is extremely unbalanced. It takes 8 calories in to get 1 calorie out of a chicken, 11 calories to get 1 calorie out of a pig and 34 calories to get 1 calorie out of a cow. Alternative protein sources, on the other hand, have an average of a 1:1 calorie conversion. It takes years to grow animals but only hours to grow microbes.
“This is the underlying weakness in the animal protein system that leads to a lot of the negative externalities that we focus on and really need to be solved as part of our protein system,” Weston says. “We have to ameliorate these effects, we have to find ways to mitigate these risks and avoid some of these negative externalities associated with the way in which we currently produce industrialized animal proteins.”
What are Fermented Alternative Proteins?
Alternative proteins are either plant-based and fermented using microbes or cultivated directly from animal cells. Fermented proteins are made using one of three production types: traditional fermentation, biomass fermentation or precision fermentation.
“Fermentation is something familiar to most of us, it’s been used for thousands and thousands of years across a wide variety of cultures for a wide variety of foods,” Weston says, citing foods like cheese, bread, beer, wine and kimchi. “That indeed is one of the benefits for this technology, it’s relatively familiar and well known to a lot of different consumers globally.”
- Traditional fermentation refers to the ancient practice of using microbes in food. To make protein alternatives, this process uses “live microorganisms to modulate and process plant-derived ingredients.” Examples are fermenting soybeans for tempeh or Miyoko’s Creamery using lactic acid bacteria to make cheese.
- Biomass fermentation involves growing naturally occurring, protein-dense, fast-growing organisms. Microorganisms like algae or fungi are often used. For example, Nature’s Fynd and Quorn …mycelium-based steak.
- Precision fermentation uses microbial hosts as “cell factories” to produce specific ingredients. It is a type of biology that allows DNA sequences from a mammal to create alternative proteins. Examples are the heme protein in an Impossible Foods’ burger or the whey protein in Perfect Day’s vegan dairy products.
Despite fermentation’s roots in ancient food processing traditions, using it to create alternative proteins is a relatively new activity. About 80% of the new companies in the fermented alternative protein space have formed since 2015. New startups have focused on precision fermentation (45%) and biomass fermentation (41%). Traditional fermentation accounts for a smaller piece of the category (14%). There were more than 260 investors in the category in 2020 alone.
“It’s really coming onto the radar for a lot of folks in the food and beverage industry and within the alternative protein industry in a very big way, particularly over the past couple of years,” Weston says. “This is an area that the industry is paying attention too. They’re starting to modify working some of its products that have traditionally maybe been focused on dairy animal-based dairy substrates to work with plant protein substrates.”
Can Alternative Protein Help the Food System?
Fermentation has been so appealing, he adds, because “it’s a mature technology that’s been proven at different scales. It’s maybe different microbes or different processes, but there’s a proof of concept that gives us a reason to think that that there’s a lot of hope for this to be a viable technology that makes economic sense.”
GFI predicts more companies will experiment with a hybrid approach to fermented alternative proteins, using different production methods.
Though plant-based is still the more popular alternative protein source, plant-based meat has some barriers that fermentation resolves. Plant-based meat products can be dry, lacking the juiciness of meat; the flavor can be bean-like and leave an unpleasant aftertaste; and the texture can be off, either too compact or too mushy.
Fermented alternative proteins, though, have been more successful at mimicking a meat-like texture and imparting a robust flavor profile. Weston says taste, price, accessibility and convenience all drive consumer behavior — and fermented alternative proteins deliver in these regards.
And, compared to animal meat, alternative proteins are customizable and easily controlled from start to finish. Though the category is still in its early days, Weston sees improvements coming quickly in nutritional profiles, sensory attributes, shelf life, food safety and price points coming quickly.
“What excites us about the category is that we’ve seen a very strong consumer response, in spite of the fact that this is a very novel category for a lot of consumers,” Weston says. “We are fundamentally reassembling meat and dairy products from the ground up.”
- Published in Business, Food & Flavor, Health, Science
Measuring and Monitoring Fermented Food Microbiomes
Analyzing the microbiome of a fermented food will help manage product quality and identify the microbes that make up the microscopic life. Though diagnostic techniques are still developing, they’re getting cheaper and faster.
“Why should we measure the microbial composition of fermented foods? If you can make a great batch of kimchi or make awesome sourdough bread, who cares what microbes are there,” says Ben Wolfe, PhD, associate professor at Tufts University. “But when things go bad, which they do sometimes when you’re making a fermented food, having that microbial knowledge is essential so you can figure out if a microbe is the cause.”
Wolfe and Maria Marco, PhD, professor at University of California, Davis, presented on Measuring and Monitoring Fermented Food Microbiomes during a TFA webinar. Both are members of the TFA Advisory Board. During the joint presentation, the two gave an overview on microbiome analysis techniques, such as culture-dependent and culture-independent approaches.
Measuring Microbial Composition
Wolfe says there are three reasons to measure the microbial composition of fermented foods: baseline knowledge, quality control and labelling details.
“Just telling you what is in that microbial black box that’s in your fermented food that can maybe be really useful for thinking about how you could potentially manipulate that system in the future,” he says.
What can you measure in a fermented food? First there’s structure, which can determine the number of species, abundance of microbes and the different types. And second is function, which can suggest how the food will taste, gauge how quickly it will acidify and help identify known quality issues.
Studying these microorganisms — unseen by the naked eye — is done most successfully through plating in petri dishes. This technique was developed in the late 1800s
“This allowed us to study microorganisms at a single cell level to grow them in the laboratory and to really begin to understand them in depth,” Marco says. “This culture-based method, it remains the gold standard in microbiology today.”
However, there’s been a “plating bias since the development of the petri dish,” she says. Science has focused on only a select few microbes, “giving us a very narrow view of microbial life.” Fewer than 1% of all microbes on earth are known.
The microbes in fermented foods and pathogens have been studied extensively.“Over these 150 years we have now a much better understanding of the processes needed to make fermented foods, not just which microbes are these but what is their metabolism and how does that metabolism change the food to give the specific sensory safety health properties of the final product,” she says
Marco and Wolfe both shared applications of these testing techniques from research at their respective universities.
Application: Olives
At UC Davis, Marco and her colleagues studied fermented olives. Using culture-based methods, they found that the microbial populations in the olives change over time. When the fruits are first submerged for fermentation, there’s a low number of lactic acid bacteria on them — but within 15 days, these microbes bloom to 10-100 million cells per gram.
Marco was called back to the same olive plant in 2008 because of a massive spoilage event. The olives smelled and tasted the same, but had lost their firmness.
Using a culture-independent method to further study what microbes were on the defective olives, she discovered a different microbiota than on normal ones, with more bacteria and yeast.
The culprit was a yeast.“Fermented food spoilage caused by yeast is difficult to prevent,” Marco says. “New approaches are needed.”
Application: Sourdough
At Tufts, Wolfe was one of the leaders on a team of scientists from four different universities that studied 500 sourdough starters with an aim to determine microbial diversity. Starters from four continents were examined in the first sourdough study encompassing a large geographic region.
The research team identified a large diversity among the starters, attributed to acetic acid bacteria. They also found geography doesn’t influence sourdough flavor.
“Everyone talks about how San Francisco sourdough is the best, which it is really great, but in our study we found no evidence that that’s driven by some special community of microbes in San Francisco,” Wolfe said. “You can find the exact same sourdough biodiversity based on our microbiome sequencing in San Francisco that you can find here in Boston or you could find in France or in any part of the world, really.”
Wolfe and Marco will return for another TFA webinar on July 14, Managing Microbiomes to Control Quality.
- Published in Science
Advances in Yeast
We’re in the midst of a yeast revolution, as genome sequencing creates opportunity for cutting-edge advances in fermented foods and drinks. Yeast will be at the forefront of innovation in fermentation, for new flavors, better quality and more sustainability.
“Understanding and respecting tradition is a key part of this. These practices have been tested for hundreds and thousands of years and they cannot be dismissed. There’s a lot the science can learn from tradition,” says Richard Preiss of Escarpment Laboratories. Priess was joined by Ben Wolfe, PhD, associate professor at Tufts University (and TFA Advisory Board member), during a TFA webinar, Advances in Yeast.
Preiss continues: “There’s still a place for innovation, despite such a long history of tradition with fermentation. A lot of the key advances in science are literally a result of people trying to make fermentation better.”
Wolfe, who uses fermented foods and other microbial communities to study microbiomes in his lab at Tufts, said “there’s this tradition versus technology conflict that can emerge.”
“I tell my students when I teach microbiology that much of the history of microbiology is food microbiology, it is actually food microbes, and they really drove the innovation of the field so it really all comes back to food and fermentation,” Wolfe says.
The technology relating to the yeasts used in fermentation has expanded enormously over the last decade, due heavily to advances in genome sequencing. Studying genetics allows labs like the ones Priess and Wolfe run to find the genetic blueprint of an organism and apply it to yeast. Drilling down further, they can tie genotype to phenotype to determine characteristics of a yeast strain. This rapidly expanding technology will disrupt and advance fermentation.
Priess predicted three areas of development for yeast fermentation in the coming years:
- Novelty Strains
Consumers have accelerated their acceptance of e-commerce during the Covid-19 pandemic and they’ll do the same for biotechnology, Priess says.
“Our industry does thrive on novelty,” he adds, noting there are beer brands already creating drinks with GMO yeast. “Craft beer is going to be the first food space where the use of GMOs is widespread — we’re seeing that play out a lot faster than I ever thought it would be with some of these products already on the market. Novelty does have value.”
Wolfe noted many consumers shudder at the idea of a GMO food or beverage, but microbes in beer are dead. Consumers are not drinking a living GMO in beer.
Yeasts also already pick up new genetic material naturally, through a process called gene transfer.
“It’s part of the evolutionary process that all microbes go through,” Wolfe says. “From my own lab and from other labs, cheese and sauerkraut and all these other fermented foods are showing so much genetic exchange that’s already happening.”
- Climate Change
The food industry must address growing concerns about climate change. Priess predicts breeding plants — like barley, hops and grapes — that are more drought-tolerant, or even using yeast technologies to increase yields or the rate of fermentation.
“Craft beer is massively wasteful,” Priess says. It takes between three to seven barrels of water to make one barrel of beer. “It is something we’re going to have to reckon with the next 10 years.”
Yogurt and cheese, too, produce large amounts of waste products.
- Ease of Genomics
The cost and time of genome sequencing has reduced significantly. It used to cost thousands of dollars and take many weeks to document a yeast genome. Now, it can be done for $200 in only a few days.
“The tools to deal with the data and get some meaning from it have never been more accessible. It’s incredibly powerful,” Priess says. “We’re developing solutions for products without millions of dollars.”
Priess does not agree with companies patenting yeasts, “it’s murky territory.” He believes fermentation and science should be about collaboration, not ownership and protection.
“Working with brewers and other fermentation enthusiasts, it’s this incredibly open and collaborative space compared to a lot of the industries,” he says. “I think that’s like our secret weapon or our secret value is that fermentation is so open in terms of access to knowledge as well as in terms of people being willing to experiment and try new things. That’s how it’s able to develop so quickly.”
- Published in Food & Flavor, Science
What’s the Actual Probiotic Count of Commercial Kefir?
A new peer-reviewed study from researchers at the University of Illinois and Ohio State University found 66% of commercial kefir products overstated probiotic count and “contained species not included on the label.”
Kefir, widely consumed in Europe and the Middle East, is growing in popularity in the U.S. Researchers examined the bacterial content of five kefir brands. Their results, published in the Journal of Dairy Science, challenge the “probiotic punch” the labels claim.
“Our study shows better quality control of kefir products is required to demonstrate and understand their potential health benefits,” says Kelly Swanson, professor in human nutrition in the Department of Animal Sciences and the Division of Nutritional Sciences at the University of Illinois. “It is important for consumers to know the accurate contents of the fermented foods they consume.”
Probiotics in fermented products are listed in colony-forming units (CFUs). The more probiotics, the greater the health benefit.
According to a news release from the University of Illinois: “Most companies guarantee minimum counts of at least a billion bacteria per gram, with many claiming up to 10 or 100 billion. Because food-fermenting microorganisms have a long history of use, are non-pathogenic, and do not produce harmful substances, they are considered ‘Generally Recognized As Safe’ (GRAS) by the U.S. Food and Drug Administration and require no further approvals for use. That means companies are free to make claims about bacteria count with little regulation or oversight.”
To perform the study, the researchers bought two bottles of each of five major kefir brands. Bottles were brought to the lab where bacterial cells were counted and bacterial species identified. Only one of the brands studied had the amount of probiotics listed on its label.
“Just like probiotics, the health benefits of kefirs and other fermented foods will largely be dependent on the type and density of microorganisms present,” Swanson says. “With trillions of bacteria already inhabiting the gut, billions are usually necessary for health promotion. These product shortcomings in regard to bacterial counts will most certainly reduce their likelihood of providing benefits.”
The news release continues:
When the research team compared the bacteria in their samples against the ones listed on the label, there were distinct discrepancies. Some species were missing altogether, while others were present but unlisted. All five products contained, but didn’t list, Streptococcus salivarius. And four out of five contained Lactobacillus paracasei.
Both species are common starter strains in the production of yogurts and other fermented foods. Because those bacteria are relatively safe and may contribute to the health benefits of fermented foods, Swanson says it’s not clear why they aren’t listed on the labels.
Although the study only tested five products, Swanson suggests the results are emblematic of a larger issue in the fermented foods market.
“Even though fermented foods and beverages have been important components of the human food supply for thousands of years, few well-designed studies on their composition and health benefits have been conducted outside of yogurt. Our results underscore just how important it is to study these products,” he says. “And given the absence of regulatory scrutiny, consumers should be wary and demand better-quality commercial fermented foods.”
Fermenting — in Space?
Microbes that coexist on plants influence a crop’s size, shape, color, flavor and yield. What would these microbes do in microgravity? The International Space Station (ISS) is testing to find these results.
Flight engineer Shannon Walker (pictured) shows sample bags collected for the Grape Juice Fermentation in Microgravity Aboard ISS study. Astronauts will observe the fermentation process, measuring microbial differences. Back on earth, a matching, control sample is being observed in an environmental control chamber that mimics the ISS ambient temperature. The space and earth samples then will be analyzed for changes. Both flight and ground control samples are analyzed post-investigation for genetic change.
Read more (NASA)
“One Grain to Bind Them All”
A new study on kefir found that the individual dominant species of Lactobacillus bacteria in kefir grains cannot survive in milk on their own. The bacteria need one another to create the fermented dairy drink, “feeding on each other’s metabolites in the kefir culture.”
The research, conducted by EMBL (Europe’s laboratory for life and sciences) and Cambridge University’s Patil group and published in Nature Microbiology, illustrates a dynamic of microbes that had eluded scientists. Though scientists knew microorganisms live in communities and depend on each other to survive, “mechanistic knowledge of this phenomenon has been quite limited,” according to a press release on the research.
“Cooperation allows them to do something they couldn’t do alone,” says Kiran Patil, group leader and author of the paper. “It is particularly fascinating how L. kefiranofaciens, which dominates the kefir community, uses kefir grains to bind together all other microbes that it needs to survive — much like the ruling ring of the Lord of the Rings. One grain to bind them all.”
To make kefir, it takes a team. A team of microbes.
The group studied 15 samples of kefir, one of the world’s oldest fermented food products. Below are highlights from a press release on the published results.
A Model of Microbial Interaction
Kefir first became popular centuries ago in Eastern Europe, Israel, and areas in and around Russia. It is composed of ‘grains’ that look like small pieces of cauliflower and have fermented in milk to produce a probiotic drink composed of bacteria and yeasts.
“People were storing milk in sheepskins and noticed these grains that emerged kept their milk from spoiling, so they could store it longer,” says Sonja Blasche, a postdoc in the Patil group and an author of the paper. “Because milk spoils fairly easily, finding a way to store it longer was of huge value.”
To make kefir, you need kefir grains, which must come from another batch of kefir. They cannot be made artificially. The grains are added to milk, to ferment and grow. Approximately 24 to 48 hours later (or, in the case of this research, 90 hours later), the kefir grains have consumed the available nutrients. The grains have grown in size and number, and are removed and added to fresh milk — to begin the process anew.
For scientists, kefir is more than just a healthy beverage: it’s an easy-to-culture model microbial community for studying metabolic interactions. And while kefir is quite similar to yogurt in many ways – both are fermented or cultured dairy products full of ‘probiotics’ – kefir’s microbial community is far larger, including not just bacterial cultures but also yeast.
A “Goldilocks Zone”
While scientists know that microorganisms often live in communities and depend on their fellow community members for survival, mechanistic knowledge of this phenomenon has been quite limited. Laboratory models historically have been limited to two or three microbial species, so kefir offers – as Patil describes – a ‘Goldilocks zone’ of complexity that is not too small (around 40 species), yet not too unwieldy to study in detail.
Blasche started this research by gathering kefir samples from several sources. Though most were obtained in Germany, they may have originated elsewhere, grown from kefir grains passed down through the years..
“Our first step was to look at how the samples grow. Kefir microbial communities have many member species with individual growth patterns that adapt to their current environment. This means fast- and slow-growing species and some that alter their speed according to nutrient availability,” Blasche says. “This is not unique to the kefir community. However, the kefir community had a lot of lead time for coevolution to bring it to perfection, as they have stuck together for a long time already.”
Cooperation is key
Finding out the extent and nature of cooperation among kefir microbes was far from straightforward. Researchers combined a variety of state-of-the-art methods, such as metabolomics (studying metabolites’ chemical processes), transcriptomics (studying the genome-produced RNA transcripts) and mathematical modelling. These processes revealed not only key molecular interaction agents like amino acids, but also the contrasting species dynamics between grains and milk.
“The kefir grain acts as a base camp for the kefir community, from which community members colonise the milk in a complex yet organised and cooperative manner,” Patil says. “We see this phenomenon in kefir, and then we see it’s not limited to kefir. If you look at the whole world of microbiomes, cooperation is also a key to their structure and function.”
In fact, in another paper from Patil’s group (in collaboration with EMBL’s Bork group) in Nature Ecology and Evolution, scientists combined data from thousands of microbial communities across the globe – from in soil to in the human gut – to understand similar cooperative relationships. In this second paper, the researchers used advanced metabolic modelling to show that the co-occurring groups of bacteria, groups that are frequently found together in different habitats, are either highly competitive or highly cooperative. This stark polarization hadn’t been observed before, and sheds light on evolutionary processes that shape microbial ecosystems. While both competitive and cooperative communities are prevalent, the cooperators seem to be more successful in terms of higher abundance and occupying diverse habitats — stronger together!
The New Definition of Fermented Food
After Dr. Bob Hutkins finished a presentation on fermented foods during a respected nutrition conference, the first audience question was from someone with a PhD in nutrition: “What are fermented foods?”
“I thought ‘Doesn’t everyone know what fermentation is?’ I realized, we do need a definition. Those of us that work in this field know what we’re talking about when we say fermented foods, but even people trained in foods do not understand this concept,” says Hutkins, a professor of food science at the University of Nebraska-Lincoln. He presented The New Definition of Fermented Foods during a webinar with TFA.
Hutkins was part of a 13-member interdisciplinary panel of scientists that released a consensus definition on fermented foods. Their research, published this month in Nature Reviews Gastroenterology & Hepatology, defines fermented foods as: “foods made through desired microbial growth and enzymatic conversions of food components.”
“We needed a definition that conveyed this simple message of a raw food turning into a fermented food via microorganisms,” Hutkins says. “It brings some clarity to many of these issues that, frankly, people are confused about.”
David Ehreth, president and founder of Alexander Valley Gourmet, parent company of Sonoma Brinery (and a TFA Advisory Board member), agreed that an expert definition was necessary.
“As a producer, and having started this effort to put live culture products on the standard grocery shelf, I started doing it as a result of unique flavors that I could achieve through fermentation that weren’t present in acidified products,” Ehreth says. “Since many of us put this on our labels, we should be paying close attention to what these folks are doing, since they are the scientific backbone of our industry.”
Hutkins calls fermented foods “the original shelf-stable foods.” They’ve been used by humankind for over thousands of years, but have mushroomed in popularity in the last 15. Fermented foods check many boxes for hot food trends: artisanal, local, organic, natural, healthy, flavorful, sustainable, innovative, hip, funky, chic, cool and Instagram-worthy.
Nutrition, Hutkins hypothesizes, is a big driver of the public’s interest in fermentation. He noted that Today’s Dietitian has voted fermented foods a top superfood for the past four years.
Evidence to make bold claims about the health benefits of fermentation, though, is lacking. Hutkins says there is observational and epidemiological evidence. But randomized, human clinical trials — “the highest evidence one can rely on” — are few and small-scale for fermented foods.
Hutkins shared some research results. One study found that Korean elders who regularly consume kimchi harbor lactic acid bacteria (LAB) in their GI tract, providing compelling evidence that LAB survives digestion and reaches the gut. Another study of cultured dairy products, cheese, fermented vegetables, Asian fermented products and fermented drinks found that most contain over 10 million LAB per gram.
Still, the lack of credible studies is “a barrier we have to get past,” Hutkins says. There are confirmed health benefits with yogurt and kefir, but this research was funded by the dairy industry, a large trade group with significant resources.
“I think there’s enough evidence — most of it through these associated studies — to warrant this statement: fermented foods, including those that contain live microorganisms, should be included as part of a healthy diet.”
Are Fermented Foods Probiotics?
Probiotics and fermented foods are not equivalent, says Mary Ellen Sanders, PhD and executive science officer of the International Scientific Association of Probiotics & Prebiotics (ISAPP). She advises fermented food producers that don’t meet the criteria of a probiotic to use descriptors such as “live active cultures” or “fermented food with live microbes” on their labels rather than “probiotic.”
“There are quite a few differences between probiotics and many fermented foods. You cannot assume a fermented food is a probiotic food even if it has live cultures present,” says Sanders. She highlighted her 30 years worth of insight into the field during a TFA webinar, Are Fermented Foods Probiotics?
Some fermented foods do meet these criteria, such as some yogurts and cultured milks that are well-studied. But many traditional fermented foods do not.
Using multiple peer-reviewed scientific studies and conclusion from expert panels in the fields of probiotics and fermented foods, Sanders shared the ways in which fermented foods and probiotics differ:
- Health benefits
By definition, a probiotic must have a documented health benefit. Many fermented foods have not been tested for a health benefit.
“If you are interested in recommending health benefits from a fermented food in an evidence-based manner, many traditional fermented foods fall short. They don’t have the controlled randomized trials that will provide a causal link between the food and the health benefit,” she says. “A food may be nutritious, but probiotic benefits must stem from the live microbe, not the nutritional composition of the food. Otherwise you just have a nutritious food that happens to have live microorganisms in it. You don’t have a probiotic food.”
- Quality studies
In her presentation, Sanders shared multiple randomized clinical trials on human subjects with supported health evidence for probiotics. But there are few randomized, controlled studies on fermented foods. Most are cohort studies, which inherently have a higher risk of bias and cannot provide a causal link between consuming fermented foods and a health benefit.
“A strong hypothesis is not the same as proof,” Sanders says. “Evidence for probiotics must meet a higher standard than small associative studies, many of which are tracking biomarkers and not health endpoints.”
She noted, though, there are some studies on fermented milk and yogurt that show a conferred health benefit.
- Strain designation
Though many fermented foods do have live microbes, a probiotic is required to be identified to the strain level. The genus and species should also be properly named according to current nomenclature. Many fermented foods contain undefined microbial composition. Without that strain designation, one can’t tie the scientific evidence on that strain to the probiotic product.
- Microbe quantity
Another key differentiator is that probiotics must be delivered at a known quantity that matches the amount that results in a health benefit. Probiotics are typically quantified in colony forming units (or CFUs).
“A probiotic has a known effective dose. But fermented foods often contain unknown levels of microbes, especially at time of consumption,” Sanders says.
What Can Brands Do?
If food brands keep using the word probiotics as a catch-all to describe a fermented product, the term will lose its utility. Using “probiotics” on food with unsubstantiated proof of probiotics is a misuse of the term.
“When I see a fermented food that says probiotics on it, I very often think what they’re trying to communicate on that label [is that it] contains live microbes,” Sanders says, “because I’m doubting, at least some of the products I see, that they have any evidence of a health benefit. And so they’re just looking for a catchy, single word that will communicate to people that this has live microbes in it. ‘Live active cultures’ is something that resonates with people as well. So why not use that?”
Sanders encourages fermented brands to standardize the terms “live active cultures,” “live microbes,” “live microorganisms” or “fermented food with live microbes.” For products pasteurized after fermentation, there’s a term for them too: “Made with live cultures.”
Controlled human studies on fermented foods can be challenging, Sanders admits. Such studies can be difficult to properly blind, since placebos for foods are hard to design. The fermentation process affects the product taste so that study subjects may know what they are consuming. But the health benefits of fermented foods could be studied, though. She also advises producers to focus on the nutritional value of their food.
“That’s one thing that really has me excited about this concept of core benefits,” says Maria Marco, PhD, professor of food science and technology at University of California, Davis (and a member of TFA’s Advisory Board) and moderator of the webinar. “I think it kind of opens the doors to the possibility of fermented fruits and vegetables where there’s certain organisms, microorganisms that we’d expect to be there but again we need to know really if those microorganisms are needed to make those foods healthy.”