Managing Fermented Food Microbes to Control Quality
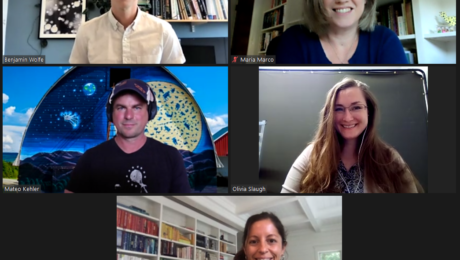
Fermented foods are produced through controlled microbial growth — but how do industry professionals manage those complex microorganisms? Three panelists, each with experience in a different field and at a different scale — restaurant chef, artisanal cheesemaker and commercial food producer — shared their insights during a TFA webinar, Managing Fermented Food Microbes to Control Quality.
“Producers of fermented foods rely on microbial communities or what we often call microbiomes, these collections of bacteria yeasts and sometimes even molds to make these delicious products that we all enjoy,” says Ben Wolfe, PhD, associate professor at Tufts University, who moderator the webinar along with Maria Marco, PhD, professor at University of California, Davis (both are TFA Advisory Board members).
Wolfe continued: “Fermenters use these microbial communities every day right, they’re working with them in crocks of kimchi and sauerkraut, they’re working with them in a vat of milk as it’s gone from milk to cheese, but yet most of these microbial communities are invisible. We’re relying on these communities that we rarely can actually see or know in great detail, and so it’s this really interesting challenge of how do you manage these invisible microbial communities to consistently make delicious fermented foods.”
Three panelists joined Wolfe and Marco: Cortney Burns (chef, author and current consultant at Blue Hill at Stone Barns in New York, a farmstead restaurant), Mateo Kehler (founder and cheesemaker at Jasper Hill in Vermont, a dairy farm and creamery) and Olivia Slaugh (quality assurance manager at wildbrine | wildcreamery in California, producers of fermented vegetables and plant-based dairy).
Fermentation mishaps are not the same for producers because “each kitchen is different, each processing facility, each packaging facility, you really have to tune in to what is happening and understand the nuance within a site,” Marco notes. “Informed trial and error” is important.
The three agreed that part of the joy of working in the culinary world is creating, and mistakes are part of that process.
“We have learned a lot over the years and never by doing anything right, we’ve learned everything we know by making mistakes,” says Kehler.
One season at Jasper Hill, aspergillus molds colonized on the rinds of hard cheeses, spoiling them. The cheesemakers discovered that there had been a problem early on as the rind developed. They corrected this issue by washing the cheese more aggressively and putting it immediately into the cellar.
“For the record, I’ve had so many things go wrong,” Burns says. A koji that failed because a heating sensor moved, ferments that turned soft because the air conditioning shut off or a water kefir that became too thick when the ferment time was off. “[Microbes are] alive, so it’s a constant conversation, it’s a relationship really that we’re having with each and every one on a different level, and some of these relationships fall to the wayside or we forget about them or they don’t get the attention they need.”
Burns continues: “All these little safeguards need to be put in place in order for us to have continual success with what we’re doing, but we always learn from it. We move the sensor, we drop the temperature, we leave things for a little bit longer. That’s how we end up manipulating them, it’s just creating an environment that we know they’re going to thrive in.”
Slaugh distinguishes between what she calls “intended microbiology” — the microbes that will benefit the food you’re creating — and “unintended microbiology” — packaging defects, spoilage organisms or a contamination event.
Slaugh says one of the benefits of working with ferments at a large scale at wildbrine is the cost of routine microbiological analysis is lower. But a mistake is stressful. She recounted a time when thousands of pounds of food needed to be thrown out because of a contaminant in packaging from an ice supplier.
“Despite the fact that the manufacturer was sending us a food-grade or in some cases a medical-grade ingredient, the container does not have the same level of sanitation, so you can’t really take these things for granted,” Slaugh says.
Her recommendations include supplier oversight, a quality assurance person that can track defects and sample the product throughout fermentation and a detailed process flow diagram. That document, Slaugh advises, should go far beyond what producers use to comply with government food regulations. It should include minutiae like what scissors are used to cut open ingredient bags and the process for employees to change their gloves.
“I think this is just an incredible time to be in fermented foods,” Kehler adds. “There’s this moment now where you have the arrival of technology. The way I described being a cheesemaker when I started making cheese almost 20 years ago was it was like being a god, except you’re blind and dumb. You’re unleashing these universes of life and then wiping them out and you couldn’t see them, you could see the impacts of your actions, but you may or may not have control. What’s happened since we started making cheese is now the technology has enabled us to actually see what’s happening. I think it’s this groundbreaking moment, we have the acceleration of knowledge. We’re living in this moment where we can start to understand the things that previously could only be intuited.”
- Published in Food & Flavor, Science
Fermentation vs. Fiber — Both Help, but Fermentation Shines
A diet high in fermented foods increases microbiome diversity, lowers inflammation, and improves immune response, according to researchers at Stanford University’s School of Medicine.The groundbreaking results were published in the journal Cell.
In the clinical trial, healthy individuals were fed for 10 weeks, a diet either high in fermented foods and beverages or high in fiber. The fermented diet — which included yogurt, kefir, cottage cheese, kimchi, kombucha, fermented veggies and fermented veggie broth — led to an increase in overall microbial diversity, with stronger effects from larger servings.
“This is a stunning finding,” says Justin Sonnenburg, PhD, an associate professor of microbiology and immunology at Stanford. “It provides one of the first examples of how a simple change in diet can reproducibly remodel the microbiota across a cohort of healthy adults.”
Researchers were particularly pleased to see participants in the fermented foods diet showed less activation in four types of immune cells. There was a decrease in the levels of 19 inflammatory proteins, including interleukin 6, which is linked to rheumatoid arthritis, Type 2 diabetes and chronic stress.
“Microbiota-targeted diets can change immune status, providing a promising avenue for decreasing inflammation in healthy adults,” says Christopher Gardner, PhD, the Rehnborg Farquhar Professor and director of nutrition studies at the Stanford Prevention Research Center. “This finding was consistent across all participants in the study who were assigned to the higher fermented food group.”
Microbiota Stability vs. Diversity
Continues a press release from Stanford Medicine News Center: By contrast, none of the 19 inflammatory proteins decreased in participants assigned to a high-fiber diet rich in legumes, seeds, whole grains, nuts, vegetables and fruits. On average, the diversity of their gut microbes also remained stable.
“We expected high fiber to have a more universally beneficial effect and increase microbiota diversity,” said Erica Sonnenburg, PhD, a senior research scientist at Stanford in basic life sciences, microbiology and immunology. “The data suggest that increased fiber intake alone over a short time period is insufficient to increase microbiota diversity.”
Justin and Erica Sonnenburg and Christopher Gardner are co-authors of the study. The lead authors are Hannah Wastyk, a PhD student in bioengineering, and former postdoctoral scholar Gabriela Fragiadakis, PhD, now an assistant professor of medicine at UC-San Francisco.
A wide body of evidence has demonstrated that diet shapes the gut microbiome which, in turn, can affect the immune system and overall health. According to Gardner, low microbiome diversity has been linked to obesity and diabetes.
“We wanted to conduct a proof-of-concept study that could test whether microbiota-targeted food could be an avenue for combatting the overwhelming rise in chronic inflammatory diseases,” Gardner said.
The researchers focused on fiber and fermented foods due to previous reports of their potential health benefits. High-fiber diets have been associated with lower rates of mortality. Fermented foods are thought to help with weight maintenance and may decrease the risk of diabetes, cancer and cardiovascular disease.
The researchers analyzed blood and stool samples collected during a three-week pre-trial period, the 10 weeks of the diet, and a four-week period after the diet when the participants ate as they chose.
The findings paint a nuanced picture of the influence of diet on gut microbes and immune status. Those who increased their consumption of fermented foods showed effects consistent with prior research showing that short-term changes in diet can rapidly alter the gut microbiome. The limited changes in the microbiome for the high-fiber group dovetailed with previous reports of the resilience of the human microbiome over short time periods.
Designing a suite of dietary and microbial strategies
The results also showed that greater fiber intake led to more carbohydrates in stool samples, pointing to incomplete fiber degradation by gut microbes. These findings are consistent with research suggesting that the microbiome of a person living in the industrialized world is depleted of fiber-degrading microbes.
“It is possible that a longer intervention would have allowed for the microbiota to adequately adapt to the increase in fiber consumption,” Erica Sonnenburg said. “Alternatively, the deliberate introduction of fiber-consuming microbes may be required to increase the microbiota’s capacity to break down the carbohydrates.”
In addition to exploring these possibilities, the researchers plan to conduct studies in mice to investigate the molecular mechanisms by which diets alter the microbiome and reduce inflammatory proteins. They also aim to test whether high-fiber and fermented foods synergize to influence the microbiome and immune system of humans. Another goal is to examine whether the consumption of fermented foods decreases inflammation or improves other health markers in patients with immunological and metabolic diseases, in pregnant women, or in older individuals.
“There are many more ways to target the microbiome with food and supplements, and we hope to continue to investigate how different diets, probiotics and prebiotics impact the microbiome and health in different groups,” Justin Sonnenburg said.
Other Stanford co-authors are Dalia Perelman, health educator; former graduate students Dylan Dahan, PhD, and Carlos Gonzalez, PhD; graduate student Bryan Merrill; former research assistant Madeline Topf; postdoctoral scholars William Van Treuren, PhD, and Shuo Han, PhD; Jennifer Robinson, PhD, administrative director of the Community Health and Prevention Research Master’s Program and program manager of the Nutrition Studies Group; and Joshua Elias, PhD.
Researchers from the nonprofit research center Chan-Zuckerberg Biohub also contributed to the study. Here’s the complete press release from Stanford Medicine News Center.
More Than One SCOBY?
A SCOBY is the gelatinous bacteria colony central to making kombucha. But did you know there are four different types of SCOBY? Scientists at Oregon State University spent the past four years researching the microorganisms that contribute to the tea fermentation that produces kombucha. The results of their work were published in the journal Microorganisms.
SCOBY is a challenging mystery to many kombucha brewers. Little is known about how SCOBY impacts flavor. The OSU scientists aim to help kombucha brewers make a more consistent product.
“Without having a baseline of which organisms are commonly most important, there are too many variables to try and think about when producing kombucha,” says Chris Curtin, an assistant professor of fermentation microbiology at OSU. “Now with this research we can say there are four main types of SCOBY. If we want to understand what contributes to differences in kombucha flavors we can narrow that variable to four types as opposed to, say, hundreds of types.”
Curtin and doctoral student Keisha Harrison used DNA sequencing to evaluate the microorganisms in 103 SCOBYs used by kombucha brewers (primarily ones in North America). The four SCOBY types each use different combinations of yeast and bacteria.
Read more (Oregon State University)
- Published in Food & Flavor, Science
Natto Stops Covid-19?
Natto — the sticky, slimy fermented soybeans, commonly eaten in Japan — inhibits infection by the coronavirus, according to the Tokyo University of Agriculture and Technology (TUAT). Researchers found that natto contains extracts that break down proteins on the surface of the coronavirus, preventing it from infecting cells.
Their results, published in the journal Biochemical and Biophysical Research Communications, note further studies are needed to determine if there are antiviral properties in the food. But the trial found natto also limited infection by Bovine herpesvirus-1 (BHV-1), a cause of outbreaks of respiratory disease in cattle around the world.
Important to note: the study was funded by Takano Foods Co., Ltd., a Japanese company that makes natto commercially.
Read more (Biochemical and Biophysical Research Communications)
Which Are Healthier — Fermented or Acidified Pickles?
Researchers with the USDA have found that fermented cucumber pickles contain more of the naturally-occurring gamma-aminobutyric acid (GABA) than do their acidified counterparts. Results of this study of commercially-available pickles were recently published in the Journal of Food Composition and Analysis.
GABA works as a neurotransmitter in the brain. It has been scientifically proven that GABA, when consumed in foods or supplements, reduces blood pressure, improves decision making, reduces anxiety and boosts immunity.
Fermented cucumber pickles undergo a lactic acid fermentation, whereas acidified cucumber pickles are submerged in an acidic brine. The fermented pickles with the most GABA were made in a low-salt fermentation, and the products were prepared for direct consumption. GABA content also was found to remain stable during storage for fermented cucumbers.
“Worldwide, people are interested in consuming fermented foods as part of a healthy lifestyle. Most often, we associate the healthfulness of fermented foods with probiotic microbes. But many fermented foods contain few to no microbes when consumed,” said Jennifer Fideler Moore, North Carolina State University graduate research assistant and one of the study co-authors, in a USDA-ARS press release. “Our research shows that the health-promoting potential of lactic acid fermented cucumbers reaches far beyond the world of probiotics. This opens the door to more research into health-promoting compounds made during fermentation of fruits and vegetables.”
Adds Suzanne Johanningsmeier, study co-author and USDA Agricultural Research Service (USDA-ARS) Research Food Technologist: “Fruits and vegetables are made up of thousands of unique molecules. These molecules rule the flavor, texture, and nutritional value, but it is difficult to study them in such complex systems. To tackle this problem, we use advanced analytical chemistry techniques like mass spectrometry to study food molecules and figure out the best food processing methods for improved quality of fruit and vegetable products.”
[Johanningsmeier presented further details of the study during a TFA webinar.]Farming with Beer Waste
In a study published in Frontiers in Sustainable Food Systems, researchers detail how they have created an eco-friendly pesticide using beer bagasse (spent beer grains), rapeseed cake (byproduct of oil extraction from seeds) and fresh cow manure.
Chemical pesticides have been proven harmful to the environment, damaging soil and water. Pesticides are also easily consumed, and many studies link their use to multiple diseases and birth defects. Researchers from the Neiker Basque Institute for Agricultural Research and Development in Spain hope that farmers will use these organic byproducts from beer production to kill parasites, preserve healthy soil and increase crop yields.
Read more (Frontiers Science News)
- Published in Science
Can Microbes Eat Plastic?
Scientists found that rumen microbes, which ferment feed in a cow’s stomach and produce fatty acids, can also break down plastics, including the common polyethylene terephthalate (PET) used in food and drink packaging. Rumen microbes are found in the rumen of cows, the largest compartment of their stomach.
These researchers hope to determine the specific enzymes used by the microbes in this process, then genetically engineer the microbes to produce them in large quantities that could then be used at an industrial scale. The study, conducted by the University of Natural Resources and Life Sciences in Vienna, is published in the journal Frontiers in Bioengineering and Biotechnology.
This is not the first bacterium found to consume plastic. Ideonella sakaiensis, in enzymes secreted by some marine organisms and in certain fungi — and used in sake fermentation — also breaks down PETs.
Read more (Live Science)
- Published in Science
A New Breed of Animal-Free Milk
Can you imagine dairy-free milk without a nut or oat? An Israeli start-up is using precision fermentation to create animal-free milk “indistinguishable from the real thing.”
Imagindairy’s technology recreates the whey and casein proteins found in a mammal’s milk. The fermentation time is quick at 3-5 days, and the final product mimics the taste and texture of traditional dairy milk, without cholesterol or GMOs. The product is expected to be in stores in the next two years.
“Many food products are produced in fermentation, including enzymes, probiotics and proteins,” says Eyal Afergan, co-founder and CEO of Imagindairy. He emphasized how safe the product is. “In fact, on the contrary, fermentation process produces a cleaner product which is antibiotic free and reduces the exposure to a potential milk borne pathogens.”
Read more (Food Navigator)
Fermentation for Alternative Proteins
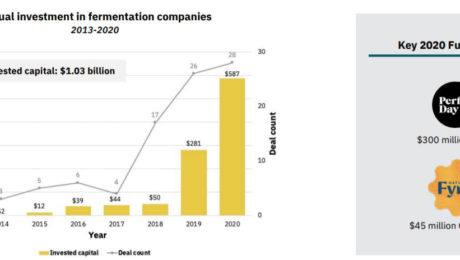
Investments in alternative protein hit their highest level in 2020: $3.1 billion, double the amount invested from 2010-2019. Over $1 billion of that was in fermentation-powered protein alternatives.
It’s a time of huge growth for the industry — the alternative protein market is projected to reach $290 billion by 2035 — but it represents only a tiny segment of the larger meat and dairy industries.
Approximately 350 million metric tons of meat are produced globally every year. For reference, that’s about 1 million Volkswagen Beetles of meat a day. Meat consumption is expected to increase to 500 million metric tons by 2050 — but alternative proteins are expected to account for just 1 million.
“The world has a very large demand for meat and that meat demand is expected to go up,” says Zak Weston, foodservice and supply chain manager for the Good Food Institute (GFI). Weston shared details on fermented alternative proteins during the GFI presentation The State of the Industry: Fermentation for Alternative Proteins. “We think the solution lies in creating alternatives that are competitive with animal-based meat and dairy.”
Why is Alternative Protein Growing?
Animal meat is environmentally inefficient. It requires significant resources, from the amount of agricultural land needed to raise animals, to the fertilizers, pesticides and hormones used for feed, to the carbon emissions from the animals.
Globally, 83% of agricultural land is used to produce animal-based meat, dairy or eggs. Two-thirds of the global supply of protein comes from traditional animal protein.
The caloric conversion ratios — the calories it takes to grow an animal versus the calories that the animal provides when consumed — is extremely unbalanced. It takes 8 calories in to get 1 calorie out of a chicken, 11 calories to get 1 calorie out of a pig and 34 calories to get 1 calorie out of a cow. Alternative protein sources, on the other hand, have an average of a 1:1 calorie conversion. It takes years to grow animals but only hours to grow microbes.
“This is the underlying weakness in the animal protein system that leads to a lot of the negative externalities that we focus on and really need to be solved as part of our protein system,” Weston says. “We have to ameliorate these effects, we have to find ways to mitigate these risks and avoid some of these negative externalities associated with the way in which we currently produce industrialized animal proteins.”
What are Fermented Alternative Proteins?
Alternative proteins are either plant-based and fermented using microbes or cultivated directly from animal cells. Fermented proteins are made using one of three production types: traditional fermentation, biomass fermentation or precision fermentation.
“Fermentation is something familiar to most of us, it’s been used for thousands and thousands of years across a wide variety of cultures for a wide variety of foods,” Weston says, citing foods like cheese, bread, beer, wine and kimchi. “That indeed is one of the benefits for this technology, it’s relatively familiar and well known to a lot of different consumers globally.”
- Traditional fermentation refers to the ancient practice of using microbes in food. To make protein alternatives, this process uses “live microorganisms to modulate and process plant-derived ingredients.” Examples are fermenting soybeans for tempeh or Miyoko’s Creamery using lactic acid bacteria to make cheese.
- Biomass fermentation involves growing naturally occurring, protein-dense, fast-growing organisms. Microorganisms like algae or fungi are often used. For example, Nature’s Fynd and Quorn …mycelium-based steak.
- Precision fermentation uses microbial hosts as “cell factories” to produce specific ingredients. It is a type of biology that allows DNA sequences from a mammal to create alternative proteins. Examples are the heme protein in an Impossible Foods’ burger or the whey protein in Perfect Day’s vegan dairy products.
Despite fermentation’s roots in ancient food processing traditions, using it to create alternative proteins is a relatively new activity. About 80% of the new companies in the fermented alternative protein space have formed since 2015. New startups have focused on precision fermentation (45%) and biomass fermentation (41%). Traditional fermentation accounts for a smaller piece of the category (14%). There were more than 260 investors in the category in 2020 alone.
“It’s really coming onto the radar for a lot of folks in the food and beverage industry and within the alternative protein industry in a very big way, particularly over the past couple of years,” Weston says. “This is an area that the industry is paying attention too. They’re starting to modify working some of its products that have traditionally maybe been focused on dairy animal-based dairy substrates to work with plant protein substrates.”
Can Alternative Protein Help the Food System?
Fermentation has been so appealing, he adds, because “it’s a mature technology that’s been proven at different scales. It’s maybe different microbes or different processes, but there’s a proof of concept that gives us a reason to think that that there’s a lot of hope for this to be a viable technology that makes economic sense.”
GFI predicts more companies will experiment with a hybrid approach to fermented alternative proteins, using different production methods.
Though plant-based is still the more popular alternative protein source, plant-based meat has some barriers that fermentation resolves. Plant-based meat products can be dry, lacking the juiciness of meat; the flavor can be bean-like and leave an unpleasant aftertaste; and the texture can be off, either too compact or too mushy.
Fermented alternative proteins, though, have been more successful at mimicking a meat-like texture and imparting a robust flavor profile. Weston says taste, price, accessibility and convenience all drive consumer behavior — and fermented alternative proteins deliver in these regards.
And, compared to animal meat, alternative proteins are customizable and easily controlled from start to finish. Though the category is still in its early days, Weston sees improvements coming quickly in nutritional profiles, sensory attributes, shelf life, food safety and price points coming quickly.
“What excites us about the category is that we’ve seen a very strong consumer response, in spite of the fact that this is a very novel category for a lot of consumers,” Weston says. “We are fundamentally reassembling meat and dairy products from the ground up.”
- Published in Business, Food & Flavor, Health, Science
Measuring and Monitoring Fermented Food Microbiomes
Analyzing the microbiome of a fermented food will help manage product quality and identify the microbes that make up the microscopic life. Though diagnostic techniques are still developing, they’re getting cheaper and faster.
“Why should we measure the microbial composition of fermented foods? If you can make a great batch of kimchi or make awesome sourdough bread, who cares what microbes are there,” says Ben Wolfe, PhD, associate professor at Tufts University. “But when things go bad, which they do sometimes when you’re making a fermented food, having that microbial knowledge is essential so you can figure out if a microbe is the cause.”
Wolfe and Maria Marco, PhD, professor at University of California, Davis, presented on Measuring and Monitoring Fermented Food Microbiomes during a TFA webinar. Both are members of the TFA Advisory Board. During the joint presentation, the two gave an overview on microbiome analysis techniques, such as culture-dependent and culture-independent approaches.
Measuring Microbial Composition
Wolfe says there are three reasons to measure the microbial composition of fermented foods: baseline knowledge, quality control and labelling details.
“Just telling you what is in that microbial black box that’s in your fermented food that can maybe be really useful for thinking about how you could potentially manipulate that system in the future,” he says.
What can you measure in a fermented food? First there’s structure, which can determine the number of species, abundance of microbes and the different types. And second is function, which can suggest how the food will taste, gauge how quickly it will acidify and help identify known quality issues.
Studying these microorganisms — unseen by the naked eye — is done most successfully through plating in petri dishes. This technique was developed in the late 1800s
“This allowed us to study microorganisms at a single cell level to grow them in the laboratory and to really begin to understand them in depth,” Marco says. “This culture-based method, it remains the gold standard in microbiology today.”
However, there’s been a “plating bias since the development of the petri dish,” she says. Science has focused on only a select few microbes, “giving us a very narrow view of microbial life.” Fewer than 1% of all microbes on earth are known.
The microbes in fermented foods and pathogens have been studied extensively.“Over these 150 years we have now a much better understanding of the processes needed to make fermented foods, not just which microbes are these but what is their metabolism and how does that metabolism change the food to give the specific sensory safety health properties of the final product,” she says
Marco and Wolfe both shared applications of these testing techniques from research at their respective universities.
Application: Olives
At UC Davis, Marco and her colleagues studied fermented olives. Using culture-based methods, they found that the microbial populations in the olives change over time. When the fruits are first submerged for fermentation, there’s a low number of lactic acid bacteria on them — but within 15 days, these microbes bloom to 10-100 million cells per gram.
Marco was called back to the same olive plant in 2008 because of a massive spoilage event. The olives smelled and tasted the same, but had lost their firmness.
Using a culture-independent method to further study what microbes were on the defective olives, she discovered a different microbiota than on normal ones, with more bacteria and yeast.
The culprit was a yeast.“Fermented food spoilage caused by yeast is difficult to prevent,” Marco says. “New approaches are needed.”
Application: Sourdough
At Tufts, Wolfe was one of the leaders on a team of scientists from four different universities that studied 500 sourdough starters with an aim to determine microbial diversity. Starters from four continents were examined in the first sourdough study encompassing a large geographic region.
The research team identified a large diversity among the starters, attributed to acetic acid bacteria. They also found geography doesn’t influence sourdough flavor.
“Everyone talks about how San Francisco sourdough is the best, which it is really great, but in our study we found no evidence that that’s driven by some special community of microbes in San Francisco,” Wolfe said. “You can find the exact same sourdough biodiversity based on our microbiome sequencing in San Francisco that you can find here in Boston or you could find in France or in any part of the world, really.”
Wolfe and Marco will return for another TFA webinar on July 14, Managing Microbiomes to Control Quality.
- Published in Science